Nitrogen gas
Nitrogen gas (N2) is a colorless, odorless, tasteless, and non-toxic gas that makes up about 78% of the Earth’s atmosphere. It is a non-flammable gas that is inert, meaning it does not react with other elements or compounds.
Sources
The largest source of this gas is the atmosphere, which contains about 78% this gas. Other sources of this gas include:
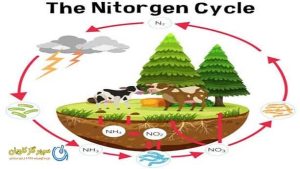
Nitrogen fixation: This is the process by which bacteria in the soil and in the roots of legumes convert atmospheric this gas into ammonia, which can be used by plants.
Fertilizers: Fertilizers can contain this gas in the form of ammonia, ammonium nitrate, or urea.
Wastewater: Wastewater treatment plants often produce nitrogen gas as a byproduct of the treatment process.
This gas can also be produced by industrial processes, such as the Haber-Bosch process. The Haber-Bosch process is a chemical reaction that combines hydrogen gas and N2 to produce ammonia. Ammonia is then used to make fertilizers, nitric acid, and other products.
The use of N2 is growing as the world’s population grows and demand for food and other products increases. It is important to manage the use of nitrogen gas responsibly to avoid environmental problems, such as water pollution and smog.

Technical Specifications
| Chemical formula | N2 |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Density | 1.168 g/L at 20°C and 1 atm |
| Boiling point | -195.8°C |
| Melting point | -210.0°C |
| Vapor pressure | 33.39 kPa at 20°C |
| Flammability limits | N/A |
| Toxicity | Non-toxic |
Application
Nitrogen gas is a versatile gas with a wide range of uses. Here are some of the most common uses of N2:
In the production of fertilizers: This gas is the main component of ammonia, which is used to make fertilizers. Fertilizers are essential for crop production, and this gas is a key ingredient in the production of fertilizers.
In the production of nitric acid: Nitric acid is used to make a variety of products, including explosives, nylon, and dyes. This gas is used to produce nitric acid by reacting it with hydrogen gas.
In cryogenics: This gas is used as a coolant in cryogenics, which is the study of matter at very low temperatures. N2 has a very low boiling point, so it can be used to cool down materials to very low temperatures.
In packaging: N2 is used to package food and other products to prevent them from spoiling. This gas is inert, so it does not react with food or other products. This helps to prevent the growth of bacteria and mold, which can spoil food.
In medical applications: This gas is used in medical procedures, such as MRI scans, to displace oxygen and prevent fires. N2 is also used as an anesthetic in some medical procedures.
These are just a few of the many uses of this gas. N2 is a valuable resource with a wide range of applications. It is important to use it safely and responsibly.
Here are some other uses of N2:
In the semiconductor industry: This gas is used to purge and flush equipment and to create an inert atmosphere for manufacturing processes.
In the food industry: This gas is used to flush out oxygen and prevent oxidation in food packaging, to create a foamy texture in some foods, and to pressurize whipped cream canisters.
In the welding industry: This gas is used as a shielding gas to prevent oxidation during welding.
In the fire suppression industry: N2 is used as an extinguishing agent in some fire suppression systems.
This gas is a valuable resource that has a wide range of applications. It is important to use it safely and responsibly
Danger
Here are some of the dangers of N2:
Asphyxiation: This gas can displace oxygen in the air, leading to suffocation. This can happen even if the nitrogen gas is not visible or odorous.
Liquefied nitrogen burns: Liquefied N2 can cause severe burns if it comes into contact with skin. The burns can occur even if the skin is not visibly wet.
Cryogenic injuries: Liquefied nitrogen can cause cryogenic injuries if it comes into contact with skin or eyes. These injuries can be very serious and can lead to permanent damage.