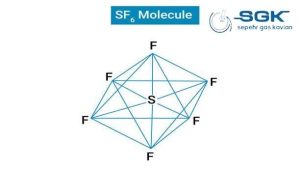
Sulfur hexafluoride gas
Sulfur hexafluoride (SF6) is a colorless, odorless, non-flammable, and non-toxic gas.
Sepher gas kavian is a producer and importer of high quality laboratory gases and gas mixtures (calibration gases) and all related equipment for various industries. Sepher gas kavian is active in supplying pure laboratory gases and mixed gases in percent, PPM, PPB such as sulfur hexafluoride gas.
Sulfur hexafluoride is available in grades 99.95, 99.99 and 99.999 in carbon steel cylinders.
Sulfur hexafluoride with a purity of 99.95 is available in a 5-liter cylinder of 2 kg and in a 10-liter cylinder of 5 kg.
Sulfur hexafluoride with a purity of 99.99 is available in a 40 liter 40 kg cylinder.
Sulfur hexafluoride with a purity of 99.999 is available in a 50 liter 50 kg cylinder.

Resources
Sulfur hexafluoride gas is a man-made gas, so there are no natural sources of it. It is produced by the reaction of sulfur with fluorine gas. The reaction is typically carried out at high temperatures and pressures.
The main producers of sulfur hexafluoride gas are China, the United States, and Japan. The gas is produced in large quantities, and the global market for this gas is worth billions of dollars.
This gas is typically sold in cylinders or tanks. It is also available in liquid form, but this is less common.
The use of sulfur hexafluoride gas is regulated in many countries. This is because Sulfur hexafluoride is a potent greenhouse gas, and its emissions can contribute to climate change.
There are a number of alternative gases that can be used in place of Sulfur hexafluoride. These alternatives are not as effective as this gas in some applications, but they have a lower GWP.
As the use of this gas is regulated more tightly, the demand for alternative gases is likely to increase. This will create new opportunities for companies that develop and produce these gases.
Technical specification
| Chemical formula | SF6 |
| Molecular weight | 146.06 g/mol |
| Density | 6.17 kg/m3 at 20°C and 1 atm |
| Melting point | -51.0°C |
| Boiling point | -63.8°C |
| Vapor pressure | 2.66 MPa at 20°C |
| Dielectric strength | 80 kV/cm at 20°C |
| Thermal conductivity | 0.011 W/m·K at 20°C |
Application
Sulfur hexafluoride is a colorless, odorless, and non-flammable gas that is used in a variety of applications. Here are some of the most common applications of Sulfur hexafluoride:
Electrical insulation: This gas is used as an insulating medium in high-voltage electrical equipment, such as circuit breakers and switchgear. It is also used as an arc quenching agent to prevent the spread of electrical arcs.
Medical imaging: This gas is used as a contrast agent in medical imaging procedures, such as CT scans and MRIs. It is also used in eye surgery to help visualize the eye’s interior.
Semiconductor manufacturing: This gas is used in the production of semiconductors. It is used as a purge gas to remove oxygen and moisture from the manufacturing environment.
Fire suppression: Sulfur hexafluoride is used as a fire suppressant in some applications. It is effective at extinguishing fires because it is a good insulator and it does not support combustion.
Etching: Sulfur hexafluoride is used as an etching gas in the manufacture of semiconductor devices. It is used to etch away the silicon substrate to create the desired pattern.
Tracer gas: This gas is used as a tracer gas in laboratory fume hood containment testing. It is used to verify the containment properties of the fume hood.

Danger
Here are some of the dangers of this gas:
Greenhouse gas: This gas is a potent greenhouse gas, with a global warming potential (GWP) of 23,500 times that of carbon dioxide. This means that a small amount of Sulfur hexafluoride can have a significant impact on the Earth’s climate.
Fire hazard: This gas is a fire suppressant, but it can also be flammable in high concentrations. This means that it is important to use SF6 in a safe manner and to avoid creating any potential sources of ignition.
Toxicity: This gas is not toxic, but it can cause irritation to the eyes and respiratory tract in high concentrations. This means that it is important to use SF6 in a well-ventilated area and to avoid breathing in large amounts of the gas.
Oxygen displacement: This gas is heavier than air, so it can displace oxygen in enclosed spaces. This can lead to suffocation if the concentration of Sulfur hexafluoride in the air is high enough.
Skin and eye contact: This gas can cause skin irritation and eye burns. If you come into contact with SF6, it is important to wash the affected area with soap and water immediately.
Inhalation: Inhaling SF6 can cause coughing, shortness of breath, and chest pain. In high concentrations, this gas can cause pulmonary edema, which is a build-up of fluid in the lungs.