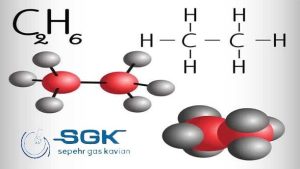
Ethane gas
Ethane gas is a colorless, odorless gas with the chemical formula C2H6. It is the second most abundant constituent of natural gas, after methane. This gas is also a by-product of oil refining and coal carbonization.
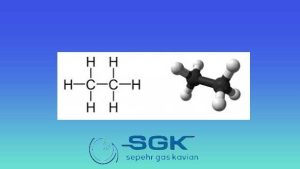
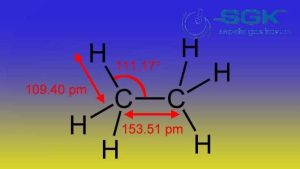
This gas is a hydrocarbon, which means that it is made up of hydrogen and carbon atoms. The carbon atoms in C2H6 are bonded together in a straight chain, with each carbon atom bonded to two hydrogen atoms.
This gas is a very flammable gas, and it is also a greenhouse gas. However, it is not as potent a greenhouse gas as methane.
Sepehr gas kavian Company is a supplier of ethane with a purity of 99.9, 99.95, 99.99.
Ethane capsules and equipment related to ethane capsules such as regulators, bundles, etc. are sold in Sepehr Gas Kavian. The volume of cylinders and capsules available for charging ethane gas in Sepehr Gas Kavian Company is: 5 liters, 10 liters, 20 liters, 40 liters, 50 liters.
The goal of Sepehr Gas Kavian Company is always to produce gases with high purity and reasonable price, according to the needs of consumers.
Resources
This gas is a naturally occurring gas that is found in a variety of sources, including:
Natural gas: C2H6 is the second most abundant constituent of natural gas, after methane. It is typically found in concentrations of 5-15% in natural gas.
Oil refinery: This gas is a by-product of oil refining. It is typically produced during the cracking process, which is used to break down larger hydrocarbon molecules into smaller ones.
Coal carbonization: This gas is also produced during the carbonization of coal. Carbonization is a process that is used to produce coke, which is a solid fuel.
Methane fermentation: This gas can also be produced by the fermentation of methane. This process is used to produce biogas, which is a mixture of methane and carbon dioxide.
C2H6 can also be produced synthetically, but this is not a common practice.
Technical specifications of Ethane gas
| Chemical formula | C2H6 |
| Molecular Weight | 30.07 g/mol |
| density | About 1.27 kg/m3 in standard conditions (temperature 0 degrees Celsius and pressure 1 atmosphere) |
| boiling point | About -88.6 degrees Celsius |
| melting point | About -183 degrees Celsius |
| steam pressure | At standard temperature, its vapor pressure is about 3.8 atmospheres. |
| Color | Colorless |
| smell | It smells like natural gas. |
Applications of Ethane gas
Used in the petrochemical industry as a fraction of that produced in the natural gas liquids plants alone.
Used in the preparation of ethanol, acetaldehyde and acetic acid which find use in paints, varnishes, adhesive, plastic etc.
Used as the most specific volatile marker for the investigation of lipid peroxidation.
Used to make ethylene, for everything from antifreeze to plastics to ripening fruit.
C2H6 is primarily used as the raw material for the production of ethylene for further production of plastics, fruit ripening and detergent making
In scientific research, it is used in liquid form for vitrifying water-rich materials
It can also be used for producing ethyl alcohol, acetic acids or other similar organic compounds
It can also be liquefied for use as fuel for automobiles
Plastic industry: Gas and its derivatives are used as raw materials in the production of various plastics. For example, in the production of polyethylene, which is one of the most widely used plastics.
Gas and energy industry: This gas is used as one of the important components of natural gas. In gas and energy industries, this gas is used to produce electricity, heating and fuel.
Chemical industry: This gas is used as a raw material in the production of various chemicals, including alcohols, aldehydes, acids and other chemical compounds.
Refineries: This gas is used as one of the important products in oil and gas refining processes. C2H6 is used as a light fuel and raw material for the production of gasoline and other petroleum products.
Dangers of Ethane gas
- Fire and explosion: This gas is a flammable fuel. If this gas explodes or ignites in inappropriate conditions, it can cause fire and explosion. Therefore, necessary safety precautions should be observed regarding the use, storage and transportation of this gas.
- Risk to health: contact with Ethane can cause side effects such as eye irritation, difficulty breathing, dizziness and even unconsciousness. Direct contact with the skin may also cause irritation and burns.
- Toxicity: This gas with high concentration and in closed environments may be toxic and cause complications such as headache, dizziness and breathing problems. Breathing the vapors of this gas in inappropriate environments can be dangerous.
- Risk to the environment: If this gas is leaked or released into the environment, it can lead to air and water pollution and pose risks to the environment and local fauna.