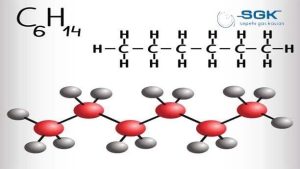
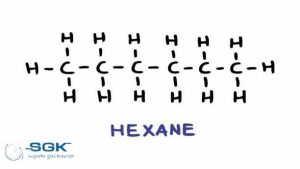
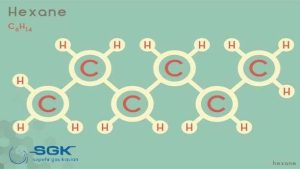
Hexane gas
Hexane gas, or so-called hexane, is a dihydrohexane (C6H14). In this compound, six carbon atoms and fourteen hydrogen atoms are connected in a chain.
Sepher gas kavian company is the reference laboratory of the standard department in the field of producing and importing oxygen, argon, etc. gases for various industries and laboratory equipment and quantum measurement devices and gas mixtures (calibration gases) with high quality and all equipment related to various active industries. is.
Resources
This gas does not exist naturally in the environment and is mostly obtained from industrial processes. The most important sources of this gas production are:
- Oil and gas industry: C6H14 is produced as a by-product in the processes of oil extraction, purification and refining. Among the main sources of production of this gas in the oil and gas industry, we can mention the processes of oil drilling, cracking of hydrocarbons, and oil refining.
- Production from natural sources: This gas is produced artificially from natural gas, methane, ammonia and other hydrocarbons. Processes such as methane reforming, hydroxycarbon completion, cracking of hydrocarbons and direct aromatics reforming are methods of producing this gas from natural sources.
- Chemical processes: In some chemical reactions, Hexane can be produced artificially. For example, hydrogenation, alkylation, hydrogenolysis and demethylation reactions can lead to the production of this gas. The most important sources of this gas production available for industrial use are crude oil, natural gas, methanol, ammonia and other hydrocarbon compounds. Hexane gas is used in various cases.
Technical specification
| Chemical formula | C6H14 |
| physical state | It exists as a colorless and odorless gas at standard temperature and pressure (25 degrees Celsius and 1 atmosphere). |
| Physical properties | The boiling point of hexane is about 69 degrees Celsius and its freezing point is about -95 degrees Celsius. Hexane is flammable at room temperature and has a strong, flaming fire. |
| Solubility | It is a strong solvent and can dissolve many substances, including oils, fats, organic matter and many chemical compounds. |
Application
This gas is used in various industries and some special applications. Below we examine some of the uses of this gas:
- Solvent: C6H14 is used as a strong solvent in various industries. For example, in the dyeing, coating and printing industry, hexane is used as a solvent for a variety of paints, resins and other chemicals.
- Extraction of oils and fats: Due to the strong solubility properties of this gas, it is used in the processes of extracting oils and fats. Hexane can extract vegetable and animal oils and fats from natural sources.
- Cleaning electronic parts: This gas is used to clean and clear electronic parts and sensitive circuits. Due to its solubility and rapid evaporation properties, Hexane is suitable for removing pollutants and oils from the surface of electronic components.
- Production of plasticizers and resins: C6H14 is used in the production of some plasticizers (materials that are made into plastic) and resins (materials used in the production of resins and adhesives).
- Cleaning materials and solvents in industry: This gas is used as a solvent in various industries such as the production of chemical products, production of varnishes, cleaning materials, perfumery and pharmaceutical industries.
- Laboratory: Hexane is used as a solvent in laboratories, especially for the extraction and separation of organic compounds in chemical research.
Danger
The use of this gas is associated with some risks and safety problems.
- Flammable: This gas is flammable and may catch fire on contact with fire sources. Therefore, it should not be used near fire, sparks or heat sources.
- Gas leakage: Hexane leakage can cause fire, explosion and other serious hazards. Therefore, you should not be exposed to leaks of this gas and any leaks should be repaired quickly and safety checked.
- Toxicity: In case of direct contact with the skin, Hexane leakage may be irritating and irritating to the skin and eyes. Side effects include irritation, burning, redness, and swelling. Therefore, safety in using and handling this gas is very important.
- Nausea and dizziness: In case of breathing high amounts of Hexane, symptoms such as nausea, dizziness, headache and drowsiness may occur. If these symptoms occur, you should be moved to an area with fresh air and see a doctor if necessary.
- Effects on the nervous system: Direct and continuous contact with this gas may have bad effects on the nervous system and cause symptoms such as headache, confusion, impaired concentration, and reduced vision. Hexane gas is used in various cases