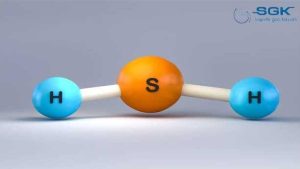
Hydrogen sulfide gas
Hydrogen sulfide (H2S) is a colorless, flammable, and corrosive gas with a characteristic rotten egg odor. It is a highly toxic gas that can be fatal at high concentrations.
Sepher gas kavian Company, reference laboratory of the standard department in the field of producer and importer of imported hydrogen sulfide gases with a purity of 99.9% in a 5-liter cylinder in the amount of 1 kg, in a 10-liter cylinder in the amount of 2 kg and in a 50-liter cylinder in the amount of 30 kg in carbon steel cylinders It is available for various industries and laboratory equipment and quantum measurement devices and gas mixture (calibration gases) with high quality and all equipment related to various industries are active.
Resources
(H2S) is a naturally occurring gas found in the Earth’s atmosphere, oceans, and soil. It is also produced by the breakdown of organic matter, such as in swamps, landfills, and sewers.
Natural sources of hydrogen sulfide
Volcanoes: Hydrogen sulfide gas is released from volcanoes as a result of the decomposition of sulfur-containing rocks.
Hot springs: This gas is released from hot springs as a result of the decomposition of organic matter in the water.
Oceans: H2S is produced in the oceans by the decomposition of organic matter.
Soil: This gas is produced in soil by the decomposition of organic matter.
Industrial sources of this gas
Oil and gas production: H2S is a common byproduct of oil and gas production.
Mining: This gas is produced in the mining of metals, such as copper and lead.
Wastewater treatment: This gas is produced in the treatment of wastewater.
PapermakingH2S is produced in the production of paper.
Technical specification
| Chemical formula | H2S |
| Molecular weight | 34.08 g/mol |
| Color | Colorless |
| Odor | Rotten eggs |
| Melting point | -85.5°C |
| Boiling point | -60.3°C |
| Density | 1.19 g/L at 20°C and 1 atm |
| Flammability | Flammable at 4.3 to 45% in air |
| Explosive range | 4.3 to 45% in air |
| Toxicity | Highly toxic |

Application
(H2S) has a variety of applications, both industrial and commercial.
Industrial applications:
Oil and gas extraction: This gas is a common byproduct of oil and gas extraction. It is used to sour crude oil, which means to remove the sulfur content from the oil.
Mining: H2S is produced in the mining of metals, such as copper and lead. It is used to extract metals from their ores.
Wastewater treatment: This gas is produced in the treatment of wastewater. It is used to kill bacteria and other microorganisms in the wastewater.
Papermaking: This gas is used in the production of paper. It is used to break down the lignin in wood pulp, which makes the pulp more pliable.
:Commercial applications
Disinfectant: This gas is a strong disinfectant. It is used to kill bacteria and other microorganisms in water, food, and other products
Fertilizer: This gas is used in the production of fertilizers. It is used to produce sulfuric acid, which is then used to make fertilizers.
Leather tanning: Hydrogen sulfide gas is used in the tanning of leather. It is used to remove the hair and fat from the leather and to make the leather more pliable.
:Other applications
Geothermal energy: Hydrogen sulfide gas is a byproduct of geothermal energy production. It is used to generate electricity and to heat homes and businesses.
Medical research: H2S is being studied for its potential therapeutic effects. It has been shown to have anti-inflammatory and analgesic effects.
Space exploration: This gas is being considered as a fuel for spacecraft. It is a clean-burning fuel that produces no emissions

Danger
:Exposure to this gas can cause a variety of health effects, including
Eye irritation: This gas can cause watering, redness, and pain in the eyes
Respiratory irritation: H2S can cause coughing, shortness of breath, and chest pain. In high concentrations, it can cause pulmonary edema, which is a build-up of fluid in the lungs
Nervous system effects: Hydrogen sulfide can cause headache, dizziness, nausea, and vomiting. In high concentrations, it can cause seizures, coma, and death
Here are some of the dangers of this gas
Highly toxic: Hydrogen sulfide is a highly toxic gas that can be fatal at high concentrations. The lethal concentration (LC50) for hydrogen sulfide is 50 ppm for 30 minutes in rats. This means that 50% of rats exposed to 50 ppm of hydrogen sulfide for 30 minutes will die
Flammable: Hydrogen sulfide is flammable at 4.3 to 45% in air. This means that it can ignite and burn if it is mixed with air in these concentrations
Corrosive: Hydrogen sulfide is corrosive to metals and can cause damage to the eyes and skin
Odorless: Hydrogen sulfide has a characteristic rotten egg odor, but this odor can be masked by other strong odors. This means that it is possible to be exposed to hydrogen sulfide without being aware of it